
Clinical Evaluation for Medical Devices
Clinical Evaluation is the assessment procedure conducted by device owners to validate whether the intended use, and the user needs for the device which can be achieved based on literatures, clinical evidence and data gathered from the clinical trial.
- The Clinical Evaluation is a part of design validation of QMS standards for Medical Device
- ISO13485 in clause 7.3.7 Design Validation,
- MDSAP in Chapter 5 : Design and Development, Design Validation.
MEDDEV 2.7.1 rev.4 is a guidance to manufacturers on how often they should prepare and update the clinical evaluation report and this guidance is also introduce the methodologies, contains additional rules on demonstrating equivalence with other medical devices and to align to the legal requirements.
Examples of related regulations
- CE Marking for Medical Device (EU MDR2017/745) ใน Annex XIV: Clinical evaluation and PMCF, Annex XV & Articles 61-82: Clinical investigation
- ASEAN Medical Device Directive (AMDD) Annex 8: Clinical Investigation
All the regulations are subjected to clinical evaluation of medical devices to validate whether the device intended use can be achieved based on clinical literatures, experience data and information gathered from the clinical trial.
- the manufacturer of medical devices should be able to reach the following conclusions through clinical evaluation;
- the medical device able to achieve the expected performance in normal condition use;
- the medical device risks are acceptably balanced with expected benefits, or its benefits should outweigh the risks in normal conditions use;
- the clinical data are sufficient evidence to support the medical device performance and safety technically.
The manufacturer requires to demonstrate the clinical data in GSPR checklist of Medical Device Regulations, MDR (Annex I) and Essential Principle checklist of Common Submission Dossier Template, CSDT (Annex I) and this is a part of Technical Documentation file and CSDT File.
Definition of Clinical Evaluation
- Refer to ISO13485:2016 (3.3): assessment and analysis of clinical data pertaining to a medical device to verify the clinical safety and performance of the device when used as intended by the manufacturer.
- Refer to MEDDEV 2.7/1 rev.4: Clinical Evaluation: a methodologically sound ongoing procedure to collect, appraise and analyze clinical data pertaining to a medical device and to evaluate whether there is sufficient clinical evidence to comply with relevant essential requirements on safety and performance when using the device according to the manufacturer’s instructions manual.
Definition of Clinical Investigation
- Systematic investigation in one or more human subjects, undertake to assess the safety or performance of a medical device. Note: 'clinical trial' or ' clinical study' are synonymous with ' clinical investigation'. [EN ISO 14155:2011]
Clinical data: (Refer to MEDDEV 2.7.1 (2016)
- The safety and/or performance information generated from the clinical use of a device. Clinical data are sourced from:
- clinical investigation(s) of the device concerned; or
- clinical investigation(s) or other studies reported in the scientific literature, of a similar device; or
- Published or unpublished reports on other clinical experience of either similar device for which equivalent to the device in query can be addressed.
Example of Clinical data: -
-
-
- Post Market Surveillance
- Any adverse effect reports
-
MEDDEV 2.7.1 rev.4
4 Stage of MEDDEV 2.7.1 rev. 4
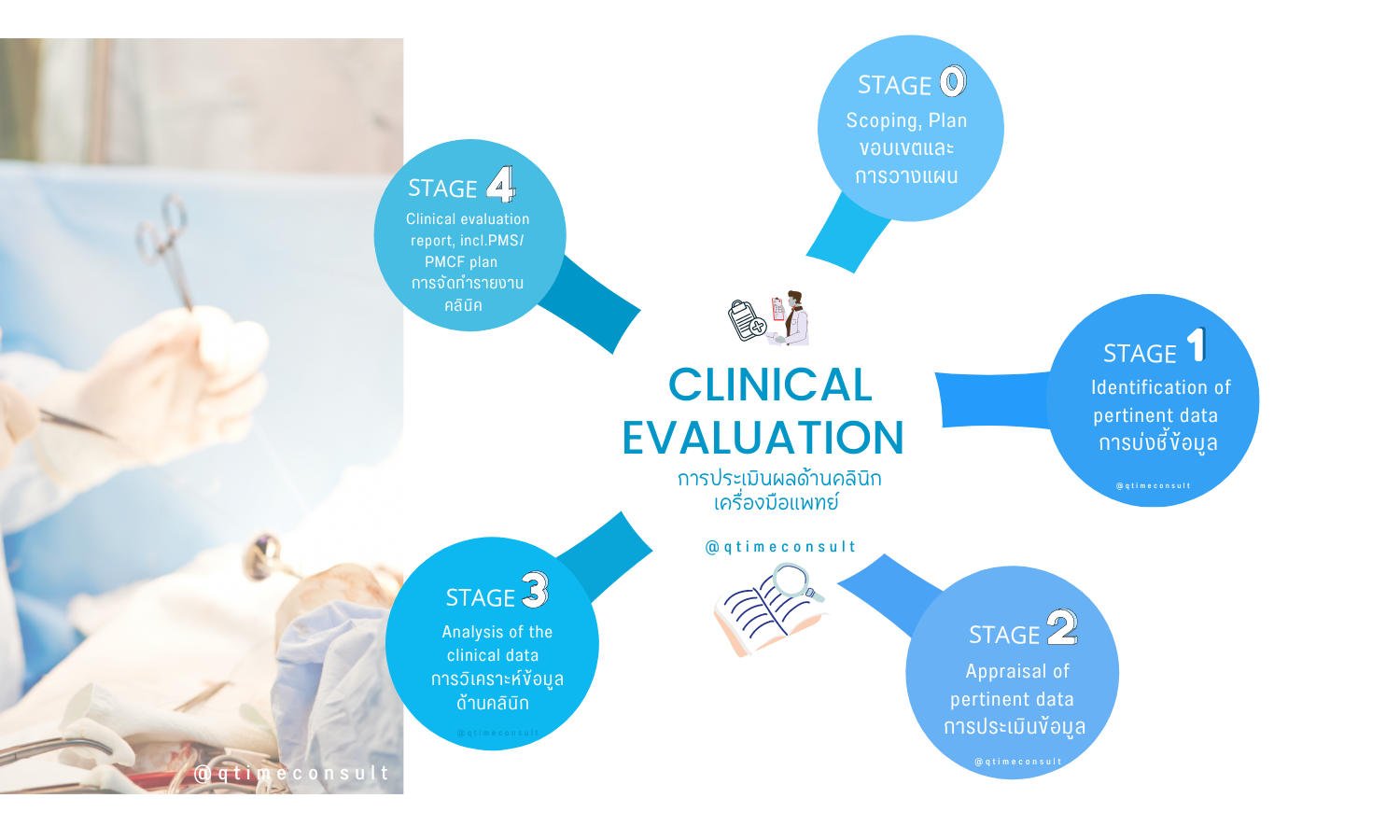
Stage 0: Define the scope of the clinical evaluation and create a plan
- To determine the scope and objectives of Clinical Evaluation through equivalent factors i.e., Biological, Technical and Clinical
- Readiness of related competent clinical evaluation team within.
Stage 1: Identify data to be appraised and literature search strategy.
- to clearly define the questions and target groups to be addressed in the assessment. The patients, interventions, comparators, outcomes, timing and setting to be evaluated have to be clearly specified..
- To determine and select the source of clinical, scientific literature or clinical trial report e.g. MEDLINE, PUBMED, ELSAVIA.
- To determine methodology of data review, collection and reference to comply with safety and performance of medical devices.
Stage 2: Review relevant data and summarize data sets.
- Collection of data from clinical trial can be obtained from public scientific literatures from the medical sources, select the appropriate data sources and methods of collection according to the characteristics of the medical device website including clinical literature and experience data
- Appraiser will have to determine the quality level of literature by using the methodology of either qualitative or quantitative. Only the highest level of literature will be selected for the analysis.
Stage 3: Perform analysis of data to see if it meets the requirements.
- The evaluator should classify the data involved in the analysis accordance to the generally accepted evaluation criteria of clinical evidence.
- Analysis and summarize the data from the highest-level literatures, whether the data is sufficient enough to support to medical device’sperformance and safety aspects.
- The appraiser should evaluate whether the device under application could meet the expected performance in normal conditions of use, and whether the risks are acceptable compared to the intended use/benefits from the analysis results from various source of data.
- To prepare risk management report highlight the benefits outweigh the risk analysis of medical device.
- To prepare and review the Instruction of use i.e., warning, potential adverse effects etc.
Stage 4: Compilation of Clinical Evaluation Report (CER)
- Clinical evaluation report preparation and covering to Stage 1 – 3
- From the result of analysis, the evaluation team should come up with the Post Market Surveillance (PMS) and Post Market Clinical Follow up Plan (PMCF).
- Signature of review and evaluation on the Clinical evaluation report
- Clinical Evaluation report shall consist of:
- Device description
- Device equivalence
- Strategy of literature approach
- Literature review, evaluation, analysis
- Post Market Surveillance (PMS)
- Post Market Clinical Follow up (PMCF)
- Benefits outweigh Risks comparison Analysis
- etc.
ในกรณีที่ต้องมีการจัดทำ Clinical Investigation ต้องปฏิบัติตามแนวทาง GCP -Good Clinical Practice ตามที่หน่วยงานกำกับดูแลประกาศ หรืออีกมาตรฐาน Guidelines : ISO14155 แนวทางปฏิบัติที่ดีสำหรับการสอบสวนทางคลินิกหรือการนำทดลองใช้กับมนุษย์ตามวัตถุประสงค์เครื่องมือแพทย์กำหนด ซึ่งการดำเนินการต้องเป็นไปตามมาตรฐานทางวิทยาศาสตร์และจริยธรรม เช่น
การคุ้มครองสิทธิและความปลอดภัยของอาสาสมัคร (Protection of Human Subjects)
ความน่าเชื่อถือและความสมบูรณ์ของข้อมูล (Data Reliability and Integrity)
การแสดงถึงความสอดคล้องกับหลักการพื้นฐานที่จำเป็น (Conformity with Essential Priciples) เรื่องประสิทธิภาพและความปลอดภัย (Performance and Safety) การชั่งน้ำหนักได้ถึงประโยชน์และความเสี่ยง (Benefit -Risks) โดยรวมในเชิงบวก หรือ Positive Overall Benefit-RiskRatio) สำหรับผู้ป่วย
บทสรุปส่งท้ายนี้ เครื่องมือแพทย์ โดยเฉพาะที่มีวัตถุประสงค์ทางการแพทย์ จำเป็นต้องได้รับการประเมินทางคลินิก (Clinical Evaluation Report -CER) ก่อน เพื่อรวบรวมและวิเคราะห์ข้อมูลทางคลินิกที่เกี่ยวข้อง และอาจจะไม่จำเป็นจำเป็นต้องดำเนินการสอบสวนทางคลินิกกับมนุษย์ (Clinical Investigation) ใหม่ แต่ทั้งนี้ขึ้นอยู่กับประกาศของหน่วยงานกำกับดูแลประกาศ หรือ Conformity Assessment Procedure ด้วย
Q Time consulting Survices :-
- Clinical Evaluation Training Click for Training course
- Clinical Evaluation Report (CER)
- Clinical Evaluation Consulting
Useful link of information and References
GUIDELINES ON MEDICAL DEVICES, MEDDEV 2.7.1 rev.4, June 2016. Clinical Evaluation. MEDDEV 2.7/1 revision 4, Clinical evaluation: a guide for manufacturers and notified bodies (medical-device-regulation.eu)
EU MDR2017/745. Chapter VI: Clinical Evaluation and Clinical Investigations, Annex XIV. REGULATION (EU) 2017/ 745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL - of 5 April 2017 - on medical devices, amending Directive 2001/ 83/ EC, Regulation (EC) No 178/ 2002 and Regulation (EC) No 1223/ 2009 and repealing Council Directives 90/ 385/ EEC and 93/ 42/ EEC (europa.eu)
IMDRF MDCE WG/N57FINAL:2019 (Formerly GHTF SG5 N3:2010 : Clinical Investigation, October 2019
MDCG 2020-6: Regulation (EU)2017/745 : Clinical Evidence needed for medical device previously CE marked under Directives 93/42/EEC or 90/385/EEC: a guide for manufacturers and notified bodies, April 2020
ASEAN Medical Device Directive
ISO 13485:2016 Medical devices — Quality management systems — Requirements for regulatory purposes
MDSAP : Medical Device Single Audit Program

